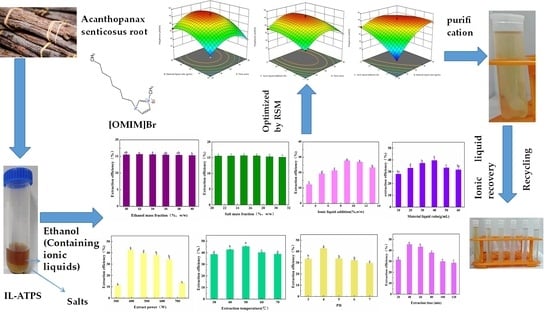
Study on Extraction and Purification of Acanthopanax senticosus Polyphenols by an Ionic Liquid-Assisted Aqueous Two-Phase System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of the Aqueous Two-Phase System
2.2. Selection of Ionic Liquids
2.3. Single-Factor Effects on IL-ATPS
- (1)
- The effect of the mass fraction of ethanol. In Figure 3a, it can be seen that the 32 wt.% mass fraction of ethanol had the highest yield. Ethanol is an essential component of ethanol-potassium dihydrogen-phosphate-based aqueous two-phase systems. Ethanol and water can be mutually dissolved, and ethanol contains hydrophobic groups and hydrophilic groups. It can affect the aqueous two-phase system’s phase-separation ability and the target components’ yield [16]. As the mass fraction of ethanol increased, the ethanol-potassium phosphate system showed an increasing and then decreasing trend in the yield of polyphenols. The possible reason is that, when the mass fraction of potassium dihydrogen phosphate is 25 wt.% and the mass fraction of ethanol is 25 wt.%, the repulsive force generated by the hydrophobic group of ethanol and the phosphate group is such that the water molecules cannot balance the affinity of the hydrophilic group and the water molecules; as such, the phase cannot be separated [11]. When the mass fraction of ethanol is increased to 32 wt.%, the hydrophilic capacity increases, and the phase-separation capacity of the solvent system also increases. At the same time, the increase in polarity in the upper phase is beneficial to the dissolution of polyphenols and is enriched in the upper phase. When the mass fraction of ethanol is higher than 40 wt.%, the mass fraction of ethanol is too large, which increases the fat solubility of the upper phase, reduces the dissolution of polyphenols, and reduces the yield [17]. Therefore, the mass fraction of ethanol is best at 32 wt.%.
- (2)
- The effect of the mass fraction of salts. Potassium dihydrogen phosphate is easily soluble in water. It exists in the system in an ionic form, which is a crucial component of the ethanol-potassium dihydrogen phosphate aqueous two-phase system [18]. In Figure 3b, with the increase in the mass fraction of the potassium dihydrogen phosphate, the yield of polyphenols in an ATPS first increases and then remains unchanged. The possible reason for this is that when the mass fraction of salt ions in the lower phase is low, the water molecules have a strong affinity for the hydrophilic groups of phosphate and ethanol; moreover, as the repulsive force between phosphate and ethanol is weak, it makes it impossible to separate the phases [18]. As the amount of salt gradually increases, the hydrophilicity of salt ions in the lower phase increases, thus promoting the system’s phase separation [19]. Therefore, changes in the amount of salt can affect the polarity of the upper phase, and ultimately, the distribution of the polyphenols therein. Thus, the mass fraction of salt was selected to be 25 wt.%.
- (3)
- The effect of the IL-ATPS. The effects of the different imidazole IL-assisted ethanol/potassium dihydrogen phosphate systems on the yield of polyphenols are shown in Figure 3c. The yield of the polyphenols by the ATPS without adding IL, which was only 5.66 mg/g, is presented in Figure S1. The addition of IL improved the yield of polyphenols to varying degrees. This is because the upper phase was ethanol-enriched in the ethanol/KH2PO4 system, and the lower phase was salt-enriched. Polyphenols tend to be distributed in the more hydrophobic and low-charge ethanol-enriched phase. The effective extraction and enrichment of the target component by ATPS were mainly the result of the balance of various forces between the elements and the target components in the two phases. The driving forces include hydrophobic interaction, hydrogen bonding, electrostatic interaction, π–π bonding conjugation, and ionic liquid self-aggregation, which means that they can enhance this driving force, and they can promote the enrichment of the target component in the corresponding phase [20]. For example, the cation structure in IL can form hydrogen bonds with polyphenols, and the π electrons on the imidazole ring can be π–π-conjugated with the π electrons of the polyphenols, thus promoting the enrichment of polyphenols in the ethanol phase. In addition, when the carbon atoms of the alkyl chain of ionic liquid are more than 6, the ionic liquid will self-aggregate and increase the yield of the polyphenols. The anionic groups in IL can accept the protons in the polyphenols [21], which is conducive to the dissolution of polyphenols and to their enrichment by the ethanol phase—this also improves the yield of polyphenols [20]. The final choice of additional ionic liquid was 9 wt.%.
- (4)
- The effect of the solid–liquid ratio. The data in Figure 3d, at the beginning, with the rise of the amount in the ATPS, show that the osmotic effect on A. senticosus powder was fully complete, and they also show that the increase in osmotic pressure will rupture the cell wall of A. senticosus, which will lead to the extraction of internal polyphenols. Finally, the water-phase solid–liquid ratio was 1:40 g/mL.
- (5)
- The effect of ultrasonic power. The data in Figure 3e show that the product was at the highest when the ultrasonic energy was 396 W. This was due to the enhancement of ultrasonic power, such that the oscillation was strengthened, which is conducive to the extraction of polyphenols.
- (6)
- The effect of ultrasonic temperature. The data in Figure 3f show that the polyphenol yield gradually increased when the ultrasonic temperature was increased from 30 °C to 50 °C. This was because with the rise in temperature, the average kinetic energy of the solvent molecules and the polyphenol molecules increased, the diffusion was enhanced, and the contact opportunities and times of the two molecules increased, thus increasing the number of polyphenols released. When the temperature exceeded 50 °C, the extraction rate of the polyphenols decreased. This was because the temperature was too high, and the solvent vaporization was serious, which hindered the continued dissolution of the polyphenols. Furthermore, it was found that a high temperature will accelerate the degradation of the phenolic substances. The optimum ultrasonic temperature was selected as 50 °C.
- (7)
- The effect of the pH solution. The effect of the pH solution on the extraction of polyphenols is shown in Figure 3g. It can be concluded that under acidic conditions, the extraction rate of the polyphenols gradually increased with the increase of pH to 4. When the pH value grew, the extraction rate of the polyphenols evidently decreased. The pH value of the ATPS was adjusted by adjusting the amounts of hydrochloric acid and sodium hydroxide. The pH can influence the electrostatic interactions between solutes and solvents, thus significantly affecting the solute partitioning behavior in ATPSs [11]. When the solution is too acidic, it promotes the hydrolysis of polyphenols and reduces the yield. When the pH is >4, the alkalinity of the solution was enhanced, and the phenolic hydroxyl group of the polyphenols was readily dissociated into oxygen anions, thus making it undergo oxidative polymerization, which produces gelatinous substances and leads to a decrease in the polyphenol content in the extraction solution. This is due to the polyphenols’ structure, which is stable in a weakly acidic environment. Therefore, a pH of 4 was chosen.
- (8)
- The effect of ultrasonic time. In Figure 3h, it can be seen that, when there was an increase in the ultrasonic time from 20 min to 120 min, the yield of the polyphenols increased. This occurred because the ultrasonic cavitation makes the polyphenols become more fully dissolved in the organic phase, thus increasing the yield of polyphenols within 20~40 min. On the other hand, the ultrasonic time was too short, and the efficiency of wall breaking was too low; as such, prolonging the ultrasonic time is beneficial in terms of increasing the yield of the polyphenols. When the ultrasonic time was over 40 min, the yield of polyphenols decreased. The possible reason for this is that when the ultrasonic time was 40 min, the polyphenols fully dissolved in the ethanol phase via the cavitation effect of the ultrasonic wave. When the ultrasonic time exceeded 40 min, the structure of the polyphenols was destroyed, and the hydroxyl group of polyphenols was oxidized; thus, the yield of polyphenols decreased. Therefore, an extraction time of 40 min was selected.
2.4. Plackett–Burman Design
2.5. Box–Behnken Response Surface Methodology
2.6. Validation Tests
2.7. Optimization of the Macroporous Resin for the Purification of Polyphenols
2.7.1. Selection of the Resins
2.7.2. Examination of the Concentration of the Adsorbent Solvent
2.7.3. Dynamic Adsorption and Desorption Tests
2.7.4. Adsorption Isotherm
2.8. Recycling of Ionic Liquid
2.8.1. Ionic Liquid Decolorization and Ionic Liquid Retention
2.8.2. Reuse of the [OMIM]Br Ionic Liquid
3. Materials and Methods
3.1. Material
3.2. Instruments
3.3. Optimization of the IL-ATPS’ Extraction of Polyphenols from A. senticosus
3.3.1. Extraction Procedure of Polyphenols
3.3.2. Determination of Polyphenols Content
3.3.3. Screening of Aqueous Two-Phase System
3.3.4. Selection of the Ionic Liquids
3.3.5. Ultrasonic-Assisted Extraction of the Polyphenols by IL-ATPS
3.3.6. Plackett–Burman Design
3.3.7. Box–Behnken Response Surface Methodology
3.4. Optimization of the Macroporous Resin for the Purification of Polyphenols from A. senticosus
3.4.1. Pretreatment of the Macroporous Resin
3.4.2. Selection of the Resins
3.4.3. Examination of the Adsorbent Solvent Concentration
3.4.4. Dynamic Adsorption and Desorption Tests
3.4.5. Adsorption Isotherm
3.5. Recycling of the Ionic Liquid
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jia, A.; Zhang, Y. A review of Acanthopanax senticosus (Rupr and Maxim.) harms: From ethnopharmacological use to modern application. J. Ethnopharmacol. 2021, 268, 113586–113603. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.-M.; Yue, G.G.-L. A review on the immunomodulatory activity of Acanthopanax senticosus and its active components. Chin. Med. 2019, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Cho, M. The Antioxidant Activity and Their Major Antioxidant Compounds from Acanthopanax senticosus and A. koreanum. Molecules 2015, 20, 13281–13295. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, H.; Huang, B.; Zheng, C.; Peng, W.; Qin, L. Acanthopanax senticosus: Review of botany, chemistry and pharmacology. Int. J. Pharm. Sci. 2011, 66, 83–97. [Google Scholar]
- Gerontakos, S.; Taylor, A. Findings of Russian literature on the clinical application of Eleutherococcus senticosus (Rupr. & Maxim.): A narrative review. J. Ethnopharmacol. 2021, 278, 114274–114287. [Google Scholar]
- Graczyk, F.; Gębalski, J. Phenolic Profile, Antioxidant, Anti-Enzymatic and Cytotoxic Activity of the Fruits and Roots of Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Molecules 2022, 27, 5579. [Google Scholar] [CrossRef]
- Hamzehzadeh, S.; Mahmoudifar, M. Liquid-liquid and solid-liquid equilibria of several PEG-based ABS with ionic liquid [C4C1im] Br as adjuvant at 298.15 K. J. Mol. Liq. 2022, 347, 118352–118365. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J. Ionic liquid-based salt-induced liquid-liquid extraction of polyphenols and anthraquinones in Polygonum cuspidatum. J. Pharm. Biomed. Anal. 2019, 163, 95–104. [Google Scholar] [CrossRef]
- Almeida, M.R.; Passos, H. Ionic liquids as additives to enhance the extraction of antioxidants in aqueous two-phase systems. Sep. Purif. Technol. 2014, 128, 1–10. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Ferreira, A.M.; Shahriari, S.; Freire, M.G.; Coutinho, J.A. Critical Assessment of the Formation of Ionic-Liquid-Based Aqueous Two-Phase Systems in Acidic Media. J. Phys. Chem. B 2011, 115, 11145–11153. [Google Scholar] [CrossRef]
- Ran, L.; Yang, C. Enhanced aqueous two-phase extraction of proanthocyanidins from grape seeds by using ionic liquids as adjuvants. Sep. Purif. Technol. 2019, 226, 154–161. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, X. Enhancement of long alkyl-chained imidazolium ionic liquids for the formation and extraction behaviour of PEG 600/(NH4)2SO4 aqueous two-phase system by complexing with Triton X-100. J. Mol. Liq. 2022, 367, 120358. [Google Scholar] [CrossRef]
- Mazan, V.; Boltoeva, M.Y. Mutual solubility of water and hydrophobic ionic liquids in the presence of hydrochloric acid. RSC Adv. 2016, 6, 56260–56270. [Google Scholar] [CrossRef]
- Ranke, J.; Othman, A. Explaining Ionic Liquid Water Solubility in Terms of Cation and Anion Hydrophobicity International. J. Mol. Sci. 2009, 10, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, L. Effect of ionic liquid based imidazolium as an additive on the formation of polymer/salt aqueous biphasic systems. J. Mol. Liq. 2018, 256, 1–8. [Google Scholar] [CrossRef]
- Santos, J.H.; e Silva, F.A. Ionic liquid-based aqueous biphasic systems as a versatile tool for the recovery of antioxidant compounds. Biotechnol. Prog. 2015, 31, 70–77. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y. Simultaneous separation and purification of total polyphenols, chlorogenic acid and phlorizin from thinned young apples. Food Chem. 2013, 136, 1022–1029. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, Q. Preliminary separation and purification of resveratrol from extract of peanut (Arachis hypogaea) sprouts by macroporous adsorption resins. Food Chem. 2014, 145, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X. Separation of epigallocatechin gallate and epicatechin gallate from tea polyphenols by macroporous resin and crystallization. Anal. Methods 2021, 13, 832–842. [Google Scholar] [CrossRef]
- Gan, J.; Zhang, X. Purification of polyphenols from Phyllanthus emblica L. pomace using macroporous resins: Antioxidant activity and potential anti-Alzheimer’s effects. J. Food Sci. 2022, 87, 1244–1256. [Google Scholar] [CrossRef]
- Ren, J.; Liao, L. Purification, Characterization, and Bioactivities of Polyphenols from Platycladus orientalis (L.) Franco. J. Food Sci. 2019, 84, 667–677. [Google Scholar] [PubMed]
- Wang, X.; Wang, S. Purification of Polyphenols from Distiller’s Grains by Macroporous Resin and Analysis of the Polyphenolic Components. Molecules 2019, 24, 1284–1301. [Google Scholar] [PubMed]
- Wang, Y.-H.; Meng, Y. The Chemical Characterization of Eleutherococcus senticosus and Ci-wu-jia Tea using UHPLC-UV-QTOF/MS International. J. Mol. Sci. 2019, 20, 475. [Google Scholar] [CrossRef]
- He, A.; Dong, B. Extraction of bioactive ginseng saponins using aqueous two-phase systems of ionic liquids and salts. Sep. Purif. Technol. 2018, 196, 270–280. [Google Scholar]
- Zhang, Y.; Sun, T.; Lu, T.; Yan, C. Extraction and separation of tungsten (VI) from aqueous media with Triton X-100-ammonium sulfate-water aqueous two-phase system without any extractant. J. Chromatogr. A 2016, 1474, 40–46. [Google Scholar]
- Ji, S.; Wang, Y. Extraction and purification of triterpenoid saponins from licorice by ionic liquid based extraction combined with in situ alkaline aqueous biphasic systems. Sep. Purif. Technol. 2020, 247, 116953–116961. [Google Scholar]
- Wang, N.; He, F. Purification of the total steroidal saponins from fenugreek seeds (Trigonella foenum-graecum L.) using aqueous two-phase system and determination of diosgenin content using micellar electrokinetic chromatography method. Nat. Prod. Res. 2018, 33, 453–456. [Google Scholar]
- Itoh, T.; Kobayashi, M. Hypoglycemic effect of hot-water extract of adzuki (Vigna angularis) in spontaneously diabetic KK-Ay mice. Nutrition 2009, 25, 134–141. [Google Scholar] [PubMed]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar]










| ATPS | Concentration of Bottom Phase (wt.%) | Concentration of Water (wt.%) | Phase Demixing and Formation Feature |
|---|---|---|---|
| ATPS1 | NaH2PO4 25 | 47 | Fast, Easy, Stable, Transparent |
| ATPS2 | (NH4)2SO4 25 | 47 | Fast, Easy, Stable, Transparent |
| ATPS3 | NaH2PO4 25 | 47 | Fast, Easy, Stable, Transparent |
| ATPS4 | (NH4)2SO4 25 | 47 | Fast, Easy, Stable, Transparent |
| ATPS5 | NaH2PO4 25 | 47 | Fast, Easy, Stable, Transparent |
| ATPS6 | (NH4)2SO4 25 | 47 | Fast, Easy, Stable, Transparent |
| ATPS | Phase Ratio (R) | Partition Coefficient (K) | Extraction Rate (Y, %) | Polyphenol Yield (mg/g) |
|---|---|---|---|---|
| Ethanol/NaH2PO4 | 3.44 | 7.04 | 96.03 | 5.66 |
| Ethanol/(NH4)2SO4 | 0.73 | 8.02 | 85.42 | 4.29 |
| PEG400/NaH2PO4 | 3.82 | 5.12 | 95.14 | 5.32 |
| PEG400/(NH4)2SO4 | 0.77 | 16.5 | 92.7 | 4.84 |
| Acetone/NaH2PO4 | 0.86 | 10.23 | 89.8 | 4.53 |
| Acetone/(NH4)2SO4 | 0.41 | 20.66 | 89.51 | 3.39 |
| ILs | Phase Ratio (R) | Partition Coefficient (K) | Extraction Rate (Y, %) | Polyphenol Yield (mg/g) |
|---|---|---|---|---|
| [C4mim]Cl | 4.111 | 16.865 | 98.58 | 6.29 |
| [C4mim]Br | 3.747 | 11.969 | 97.82 | 6.50 |
| [C4mim]BF4 | 3.330 | 12.166 | 97.59 | 5.94 |
| [BMIM]OTF | 3.002 | 8.474 | 96.22 | 6.14 |
| [BMIM]SO4 | 4.105 | 9.851 | 97.59 | 6.02 |
| [HMIM]Cl | 3.680 | 5.568 | 95.35 | 2.40 |
| [HMIM]BF4 | 3.258 | 7.732 | 96.18 | 3.62 |
| [OMIM]BF4 | 2.780 | 26.719 | 98.67 | 13.01 |
| [OMIM]Br | 2.923 | 25.337 | 98.67 | 15.90 |
| Level | Factors | ||||
|---|---|---|---|---|---|
| Temperature (A)/°C | Time (B)/min | Power (C)/W | Solid–Liquid Ratio (D)/g/mL | IL Addition Amount (E)/(g, w/w) | |
| −1 | 40 | 20 | 311 | 30 | 7 |
| 1 | 60 | 60 | 479 | 50 | 11 |
| Test Group | Temperature (A)/°C | Time (B)/min | Power (C)/W | Material–Liquid Ratio (D)/g/mL | IL Addition Amount (E)/ (wt.%) | Polyphenol Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 1 (60) | −1 (20) | −1 (311) | −1 (30) | 1 (11) | 37.231 |
| 2 | 1 (60) | 1 (60) | −1 (311) | 1 (50) | 1 (11) | 22.712 |
| 3 | 1 (60) | −1 (20) | 1 (479) | 1 (50) | 1 (11) | 14.533 |
| 4 | −1 (40) | 1 (60) | 1 (479) | −1 (30) | 1 (11) | 41.047 |
| 5 | −1 (40) | 1 (60) | −1 (311) | 1 (50) | 1 (11) | 16.091 |
| 6 | 1 (60) | 1 (60) | −1 (311) | −1 (30) | −1 (7) | 30.688 |
| 7 | 1 (60) | −1 (20) | 1 (479) | 1 (50) | −1 (7) | 10.511 |
| 8 | −1 (40) | −1 (20) | −1 (311) | −1 (30) | −1 (7) | 28.141 |
| 9 | −1 (40) | −1 (20) | −1 (311) | 1 (50) | −1 (7) | 10.316 |
| 10 | −1 (40) | 1 (60) | 1 (479) | 1 (50) | −1 (7) | 10.706 |
| 11 | 1 (60) | 1 (60) | 1 (479) | −1 (30) | −1 (7) | 36.61 |
| 12 | −1 (40) | −1 (20) | 1 (479) | −1 (30) | 1 (11) | 32.078 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1399.34 | 5 | 279.87 | 32.52 | 0.0003 ** |
| A | 16.11 | 1 | 16.11 | 1.87 | 0.2202 |
| B | 52.27 | 1 | 52.27 | 6.07 | 0.0488 * |
| C | 0.0078 | 1 | 0.0078 | 0.0009 | 0.9770 |
| D | 1218.59 | 1 | 1218.59 | 141.59 | <0.0001 ** |
| E | 112.36 | 1 | 112.36 | 13.06 | 0.0112 * |
| Residual | 51.64 | 6 | 8.61 | ||
| Cor. Total | 1450.98 | 11 |
| Coded Values | Test Group | Time (B)/min | Material–Liquid Ratio (D)/g/mL | IL Addition Amount (E)/(%, w/w) | Polyphenol Yield (%) |
|---|---|---|---|---|---|
| 9 | 1 | 40 | 30 | 7 | 27.8226 |
| 12 | 2 | 40 | 50 | 11 | 40.3325 |
| 13 | 3 | 40 | 40 | 9 | 45.0575 |
| 4 | 4 | 60 | 50 | 9 | 41.0274 |
| 11 | 5 | 40 | 30 | 11 | 40.9907 |
| 15 | 6 | 40 | 40 | 9 | 46.9714 |
| 2 | 7 | 60 | 30 | 9 | 30.3256 |
| 14 | 8 | 40 | 40 | 9 | 44.0575 |
| 1 | 9 | 20 | 30 | 9 | 28.5844 |
| 10 | 10 | 40 | 50 | 7 | 43.8745 |
| 17 | 11 | 40 | 40 | 9 | 46.5976 |
| 6 | 12 | 60 | 40 | 7 | 35.4115 |
| 3 | 13 | 20 | 50 | 9 | 37.4957 |
| 16 | 14 | 40 | 40 | 9 | 43.5878 |
| 8 | 15 | 60 | 40 | 11 | 39.0067 |
| 5 | 16 | 20 | 40 | 7 | 33.8205 |
| 7 | 17 | 20 | 40 | 11 | 38.537 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 576.29 | 9 | 64.03 | 35.20 | <0.0001 *** |
| A: Time | 6.72 | 1 | 6.72 | 3.70 | 0.0960 |
| B: Material–liquid ratio | 153.18 | 1 | 153.18 | 84.22 | <0.0001 *** |
| C: IL addition amount | 40.22 | 1 | 40.22 | 22.11 | 0.0022 ** |
| AB | 0.8015 | 1 | 0.8015 | 0.4406 | 0.5281 |
| AC | 0.3143 | 1 | 0.3143 | 0.1728 | 0.6901 |
| BC | 69.81 | 1 | 69.81 | 38.38 | 0.0004 ** |
| A2 | 163.35 | 1 | 163.35 | 89.81 | <0.0001 *** |
| B2 | 91.73 | 1 | 91.73 | 50.43 | 0.0002 ** |
| C2 | 22.89 | 1 | 22.89 | 12.59 | 0.0094 ** |
| Residual | 12.73 | 7 | 1.82 | ||
| Lack of Fit | 3.73 | 3 | 1.24 | 0.5527 | 0.6731 |
| Pure Error | 9.00 | 4 | 2.25 | ||
| Cor. Total | 589.03 | 16 |
| Resin | Surface Area (m2/g) | Average Pore Diameter (Å) | Particle Diameter (mm) | Polarity | Moisture (%) |
|---|---|---|---|---|---|
| AB-8 | 450~530 | 13~14 | 0.3~1.25 | Middle-polar | 62~72 |
| S-8 | 100~120 | 280~300 | 0.3~1.25 | Polar | 66~72 |
| X-5 | 500~600 | 290~300 | 0.3~1.25 | Non-polar | 55 |
| D101 | 500~550 | 9~10 | 0.3~1.25 | Non-polar | 65~75 |
| HPD-100 | 650~700 | 85~90 | 0.3~1.20 | Non-polar | 65~75 |
| CAD-40 | 450~500 | 50~60 | 0.25~0.84 | Middle-polar | 55~65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, X.; Wang, X.; Xue, J.; Zhang, R.; Ding, Y.; Chu, X.; Su, J. Study on Extraction and Purification of Acanthopanax senticosus Polyphenols by an Ionic Liquid-Assisted Aqueous Two-Phase System. Molecules 2023, 28, 6383. https://doi.org/10.3390/molecules28176383
Li Y, Li X, Wang X, Xue J, Zhang R, Ding Y, Chu X, Su J. Study on Extraction and Purification of Acanthopanax senticosus Polyphenols by an Ionic Liquid-Assisted Aqueous Two-Phase System. Molecules. 2023; 28(17):6383. https://doi.org/10.3390/molecules28176383
Chicago/Turabian StyleLi, Ying, Xiaoli Li, Xueyan Wang, Jiaojiao Xue, Rui Zhang, Yi Ding, Xiuling Chu, and Jianqing Su. 2023. "Study on Extraction and Purification of Acanthopanax senticosus Polyphenols by an Ionic Liquid-Assisted Aqueous Two-Phase System" Molecules 28, no. 17: 6383. https://doi.org/10.3390/molecules28176383